Patient support
|
To view the patient guide, click below. |
 About Gliolan®
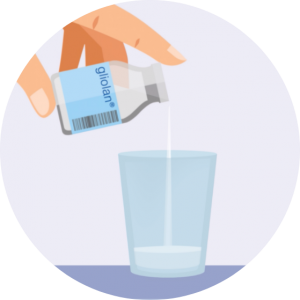
About Gliolan®Gliolan®, often called the pink drink or 5-ALA, is a valuable surgical aid for adults who are undergoing neurosurgery for high grade malignant gliomas, or glioblastomas, the most common type of primary brain tumour.
Taken as a drink, Gliolan® makes the tumour glow pink, allowing specially trained neurosurgeons to see, and remove, more of the brain tumour during surgery.
Gliolan® is a powder which is mixed with normal drinking water to give a clear liquid. It is taken as a drink around three hours before surgery to give it time to be absorbed into the bloodstream.
When it reaches the brain, it is absorbed into the tumour cells which, when viewed under an ultraviolet light, glow bright pink whist the rest of the normal brain tissue shows blue. This technique is useful as it allows the neurosurgeon to see where the edges of the tumour are so that it is easier to remove and there is less chance of leaving parts of the tumour behind.
As a result, there is less risk of damage to healthy brain tissue and the overall effect is improved quality of life with more days feeling well.
Is Gliolan® given to every brain tumour patient?
There are some types of brain tumour that aren’t suitable but the most common type of tumours, gliolblastomas, are. Each patient will be advised by their neurosurgeon.
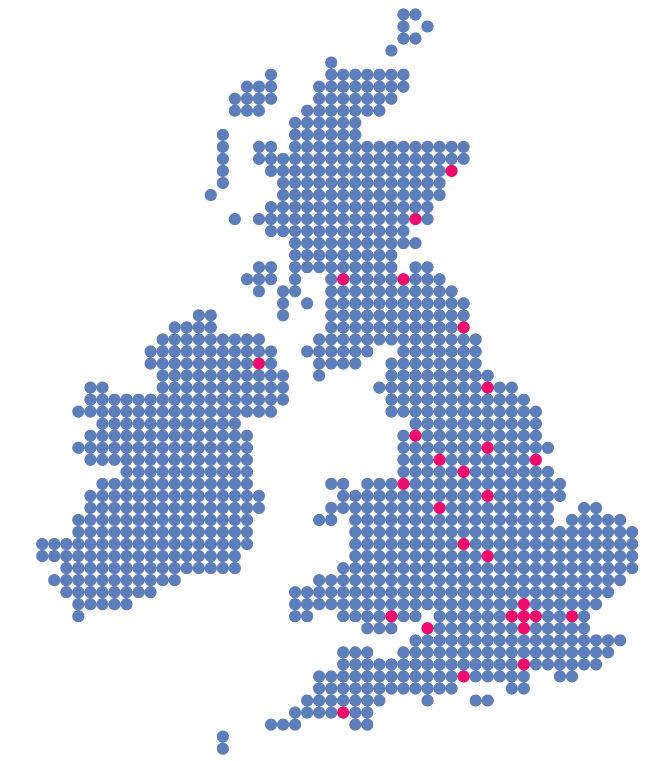
Is Gliolan® available at every neurosurgical centre in the UK?
Gliolan® should be available to patients in every neurosurgical centre in the UK and therefore anyone who could benefit from it will have access to it.
What happens if surgery is delayed?
If anaesthesia or surgery is delayed after Gliolan® has been taken, an additional dose will not be needed if the operation still takes place later the same day. However, if surgery is delayed by one or more days, another dose can be taken.
Are there any side effects from taking Gliolan®?
All medicines can cause side effects, although not everyone will experience them. The most common side effect is that skin and eyes are sensitive to sunlight and very bright artificial light for 24 hours after taking it. This means that direct sunlight should be avoided but the normal light on the surgical ward is acceptable.
Can Gliolan® be given to someone who is pregnant or breastfeeding?
It is not known whether Gliolan® can harm an unborn baby so it would not be used during pregnancy. Breastfeeding should be avoided for 24 hours after taking Gliolan® as it is not known whether it enters breast milk.
Availability and funding of
|
 |
The new Quality Standard for brain tumours and metastases in adults published by NICE on the 7th of December is... read more
BBC Two documentary Surgeons: At the Edge of Life, Series 4, Episode 4 (Aired on 2nd December 2021) showed brain... read more
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet. You can also report side effects directly via the Yellow Card Scheme at https://yellowcard.mhra.gov.uk or search for MHRA Yellow Card in Google Play or Apple App store. Please also report side effects directly to photonamic at photonamic-PhV@spm2-safety.com
By reporting side effects, you can help provide more information on the safety of this medicine.
This website uses Cookies to improve your browsing experience and to help with our marketing. You can read more about removing Cookies here: Cookie Policy
I'm fine with this