Menu
This page does not contain all information regarding this medicine. Please always consult your treating doctor for further information.
About 5-aminolevulinic acid (5-ALA)

5-ALA is a surgical aid for adults undergoing neurosurgery for high grade malignant gliomas.
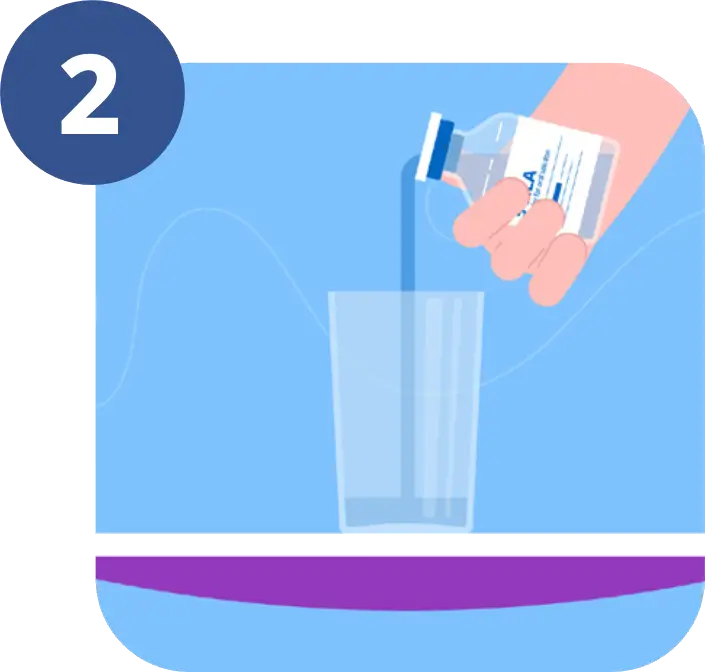
5-ALA is a powder which is mixed with drinking water to give a clear liquid.
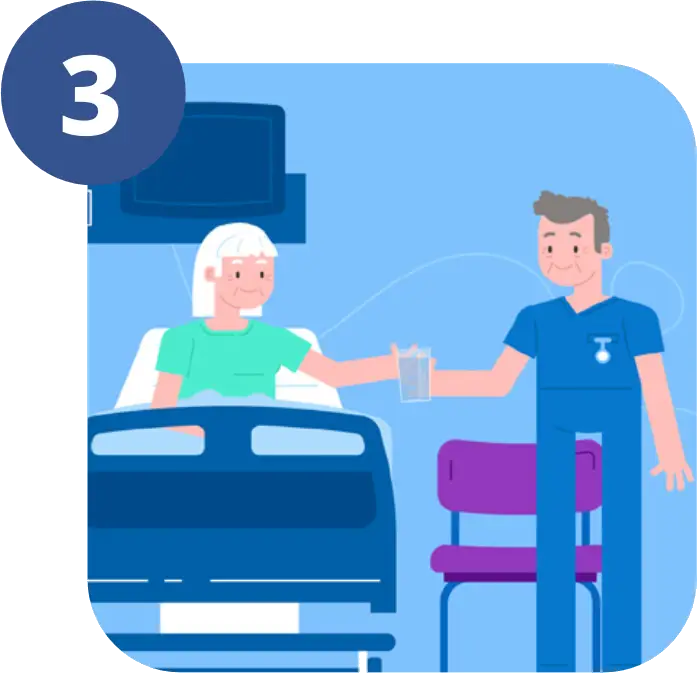
Taken as a drink you will be given it 2-4 hours before anaesthesia.

This gives it time to be absorbed into the bloodstream.
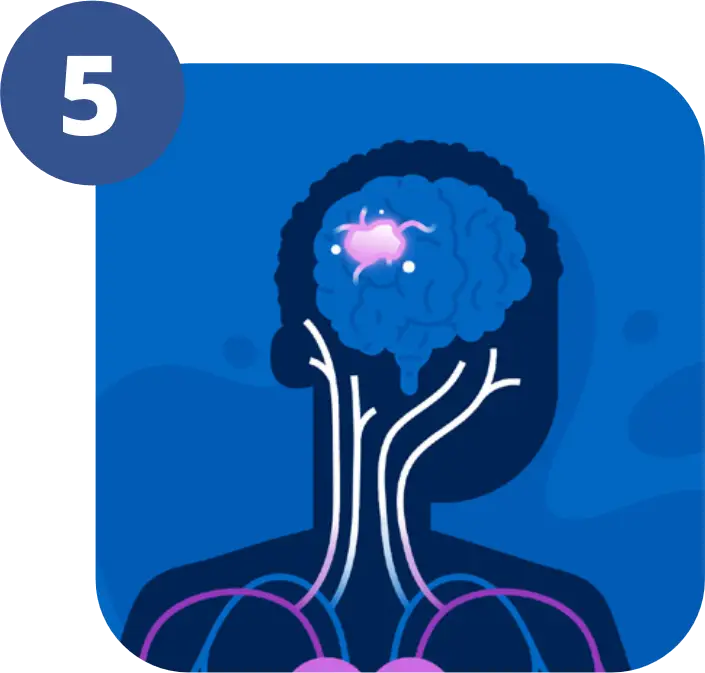
5-ALA makes the tumour glow pink under blue light.
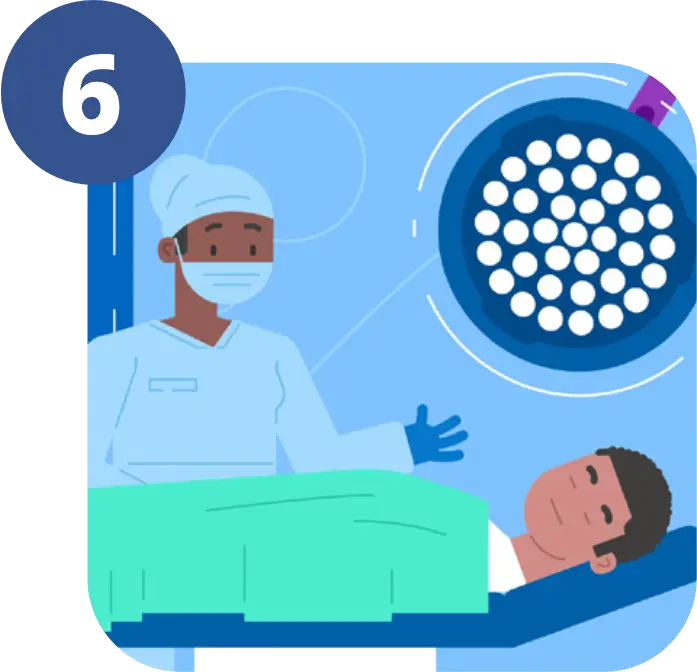
This aids specially trained neurosurgeons to better see the brain tumour during surgery, aiding with the removal.
Support
Like all medicines, 5-ALA can cause side effects, although not everyone gets them
Uncommon: Hypotension
Uncommon: Nausea
Uncommon: Photosensitivity reaction, Photodermatosis
For procedure-related side effects, these can include: Blood changes (You might have decreased red blood cells (anemia), decreased platelets (thrombocytopenia) or increased white blood cells (leukocytosis)), Vomiting, nausea, Blood clots (thromboembolism), Elevated liver enzymes and Neurological disorders.
Please ask your doctor about other rare or uncommon side effects.
Frequently asked questions
What happens after my treatment?
 After 24 hours a clinician will follow up with you regarding next steps on your recovery.
After 24 hours a clinician will follow up with you regarding next steps on your recovery.What if my surgery is delayed?
 If surgery or anaesthesia is delayed after 5-ALA has been given, an additional dose is not required, so long as the operation takes place later the same day. If surgery is delayed by more than 12 hours, you will get another dose 2-4 hours before your surgery.
If surgery or anaesthesia is delayed after 5-ALA has been given, an additional dose is not required, so long as the operation takes place later the same day. If surgery is delayed by more than 12 hours, you will get another dose 2-4 hours before your surgery.What if my surgery is cancelled?
 If surgery is cancelled there is still risk from bright light and being sunburnt so, you must remain covered for the full 24 hours after drinking 5-ALA. This includes transport and whilst at home, where lights should be kept dimmed.
If surgery is cancelled there is still risk from bright light and being sunburnt so, you must remain covered for the full 24 hours after drinking 5-ALA. This includes transport and whilst at home, where lights should be kept dimmed.Can I take 5-ALA if I am pregnant?
 It is not known whether 5-ALA will harm an unborn baby, therefore 5-ALA should not be used during pregnancy. Please talk to your clinician if you think you might be pregnant.
It is not known whether 5-ALA will harm an unborn baby, therefore 5-ALA should not be used during pregnancy. Please talk to your clinician if you think you might be pregnant.Can I breastfeed if I have had 5-ALA?
 It is not known whether 5-ALA enters breast milk. Breastfeeding should be interrupted for 24 hours after taking 5-ALA.
It is not known whether 5-ALA enters breast milk. Breastfeeding should be interrupted for 24 hours after taking 5-ALA.
Availability and funding of 5-ALA
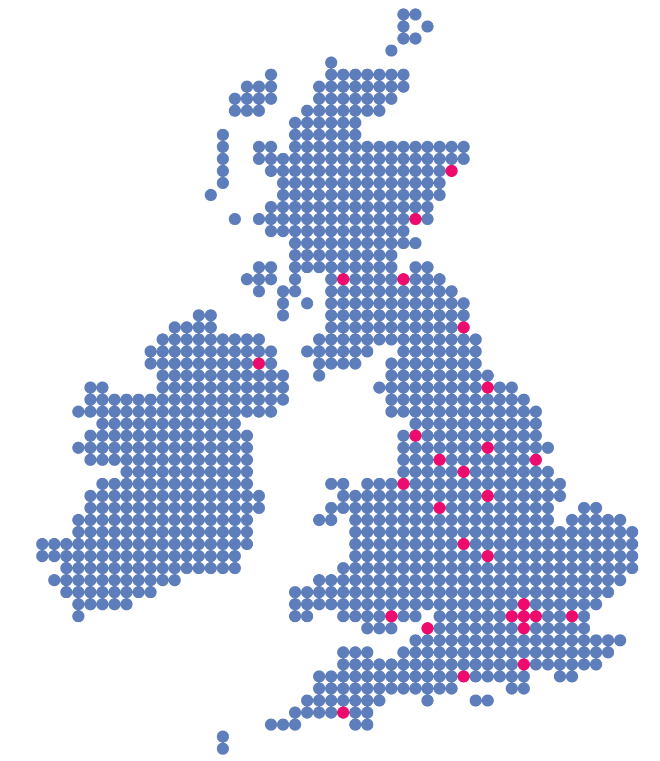
In May 2018, the UK government announced its commitment to fund a national rollout of 5-ALA. As a result, all 28 neurological centres throughout the UK should now be offering 5-ALA as standard prior to surgery, to all suitable adults with high grade gliomas.
NICE (National Institute for Health and Care Excellence) who approved the treatment stated that the roll out of 5-ALA would see more patients treated to a gold standard level of care and will help delay the recurrence of brain tumours.
If you think treatment with 5-ALA would be appropriate in your treatment, please speak to your medical team.
Side Effects
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in the package leaflet.
You can also report side effects directly via the Yellow Card Scheme at https://yellowcard.mhra.gov.uk/
By reporting side effects, you can help provide more information on the safety of this medicine.
