Menu
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk.
Adverse events should also be reported to Photonamic at: Photonamic-PhV@spm2-safety.com
NICE recommended for the visualisation of malignant tissue in adult patients with high-grade glioma1
Indication3: Gliolan® is indicated in adults for visualisation of malignant tissue during surgery for malignant glioma (WHO grade III and IV).
This medicinal product should only be used by experienced neurosurgeons conversant with surgery of malignant gliomas and in-depth knowledge of functional brain anatomy who have completed a training course in fluorescence-guided surgery.
About Gliolan®
Gliolan® is taken orally by the patient prior to surgery. In combination with a specialist surgical microscope and blue light source, Gliolan® fluoresces the malignant part of the tumour enabling the surgeon to make a distinction between malignant and normal tissue.3
Gliolan® can be used by neurosurgical units with the necessary microscopic equipment and staffed by experienced, authorised neurosurgeons in line with European Medicines Agency (EMA) regulations.4
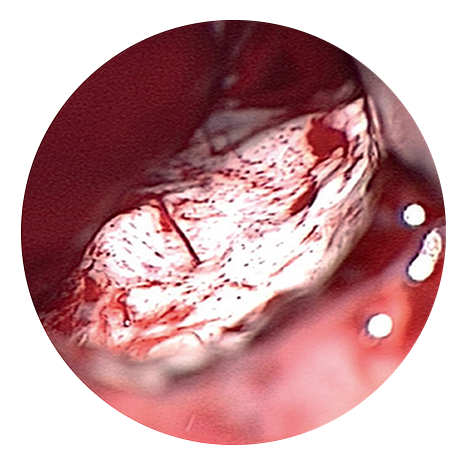
Tumour appearance under conventional light
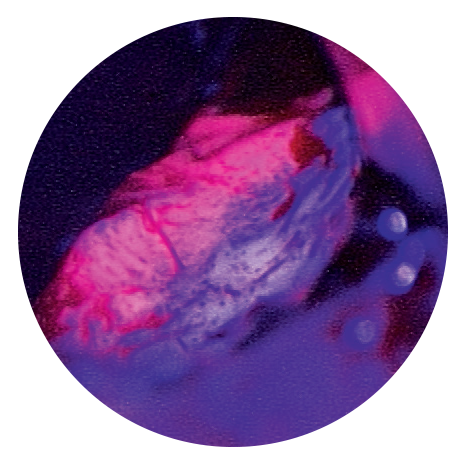
With Gliolan®, tumour tissue appears red under blue light
Using Gliolan® requires special training. For information see below
Clinical experience
Residual tumour post surgery
The result of a randomised controlled multicentre phase III trial (evidence level I) showed that by using Gliolan®, neurosurgeons achieved 64% complete resections compared to only 38% among the control group.8
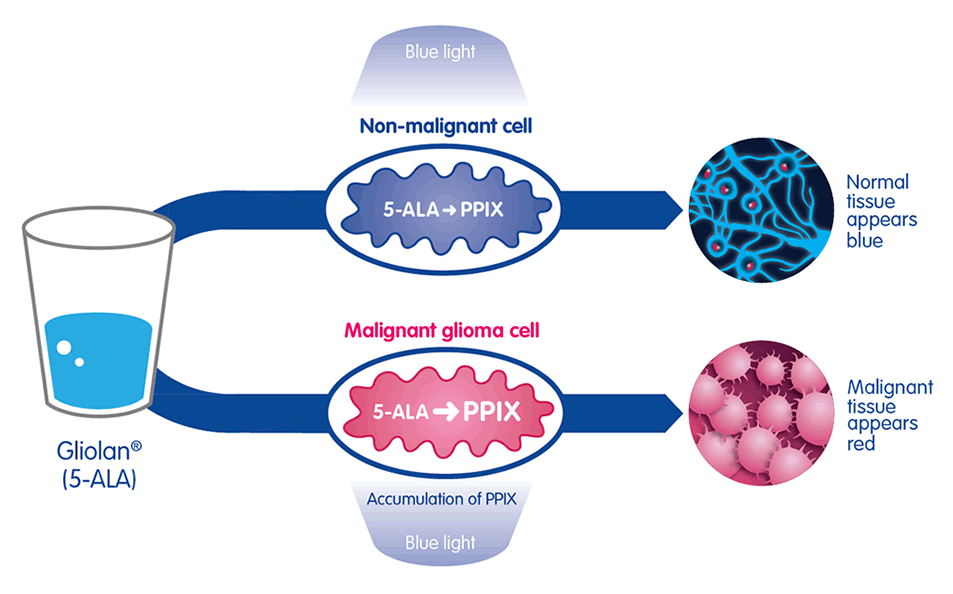
Mode of action3
The active substance in Gliolan®, 5-aminolevulinic acid (5-ALA) is absorbed by cells in the body where it is changed by enzymes into fluorescent molecules protoporphyrin IX (PPIX). Due to their different enzymes, malignant glioma cells seem to absorb and accumulate higher levels of 5-ALA than normal tissue and change it more rapidly into PPIX. When illuminated under a blue light of specific wavelength, the PPIX in the malignant tissue glows an intense red, while the normal brain tissue appears blue, enabling surgeons to distinguish between malignant and normal tissue.
Instructions for use3
- The pharmaceutical form is a powder for oral solution
- The recommended dosage is 20mg 5-aminolevulinic acid hydrochloride per kg body weight
- The solution should be administered orally three hours (range 2-4 hours) before induction of anaesthesia
Dosage scale
The interactive scale below is a guide to the correct dosage of Gliolan® and drinking solution that should be administered, depending on your patient’s bodyweight.
| Patient‘s bodyweight (kg) | Amount of Gliolan® to be administered (mg) | Volume of a freshly prepared drinking solution to be administered orally (ml) |
| 55 | 1100 | 37 |
| 56 | 1120 | 37 |
| 57 | 1140 | 38 |
| 58 | 1160 | 39 |
| 59 | 1180 | 39 |
| 60 | 1200 | 40 |
| 61 | 1220 | 41 |
| 62 | 1240 | 41 |
| 63 | 1260 | 42 |
| 64 | 1280 | 43 |
| 65 | 1300 | 43 |
| 66 | 1320 | 44 |
| 67 | 1340 | 45 |
| 68 | 1360 | 45 |
| 69 | 1380 | 46 |
| 70 | 1400 | 47 |
| 71 | 1420 | 47 |
| 72 | 1440 | 48 |
| 73 | 1460 | 49 |
| 74 | 1480 | 49 |
| 75 | 1500 | 50 |
| 76 | 1520 | 51 |
| 77 | 1540 | 51 |
| 78 | 1560 | 52 |
| 79 | 1580 | 53 |
| 80 | 1600 | 53 |
| 81 | 1620 | 54 |
| 82 | 1640 | 55 |
| 83 | 1660 | 55 |
| 84 | 1680 | 56 |
| 85 | 1700 | 57 |
| 86 | 1720 | 57 |
| 87 | 1740 | 58 |
| 88 | 1760 | 59 |
| 89 | 1780 | 59 |
| 90 | 1800 | 60 |
| 91 | 1820 | 61 |
| 92 | 1840 | 61 |
| 93 | 1860 | 62 |
| 94 | 1880 | 63 |
| 95 | 1900 | 63 |
| 96 | 1920 | 64 |
| 97 | 1940 | 65 |
| 98 | 1960 | 65 |
| 99 | 1980 | 66 |
| 100 | 2000 | 67 |
European Medicines Agency (EMA) regulations4
Gliolan® training
The use of Gliolan® improves the chances of patients receiving a complete resection of contrast enhancing tumour compared to white light.5 Thorough removal of the tumour leads to longer progression free remission time compared to control group6 and optimises effectiveness of adjuvant radiotherapy and chemotherapy.7
Before using Gliolan®, neurosurgeons must attend a training course to inform them how to use the medicine safely and effectively during surgery as required by the EMA. These are held in the following UK locations:
Addenbrooke’s Hospital, Cambridge
(Prof Stephen Price & Mr Thomas Santarius)
King’s College Hospital, London
(Prof Keyoumars Ashkan & Mr Ranj Bhangoo)
Queen Elizabeth Hospital, Birmingham
(Prof Colin Watts & Dr Victoria Wykes)
Salford Royal Hospital, Salford
(Miss Konstantina Karabatsou, Mr Pietro D’urso & Ms Helen Maye)
Courses consist of lectures and a live fluorescence guided surgery. Participants will receive a handout and an official certificate. The training follows the regulation of EMA for the appropriate use of Gliolan®.
Upcoming training courses

For details on upcoming EMA training courses and for any sales enquiries, contact your sales representative on:
E: kirk.chester@medacpharma.co.uk
T: 07825 185603
Adverse reactions observed after the use of this medicinal product are:3
Immediate reactions occurring after oral administration of the medicinal product before anaesthesia (= active substance-specific side effects)
| Cardiac disorders | Uncommon: hypotension |
| Gastrointestinal disorders | Uncommon: nausea |
| Skin and subcutaneous tissue disorders | Uncommon: photosensitivity reaction, photodermatosis |
Combined effects of 5-ALA, anaesthesia, and tumour resection (= procedure-specific side effects)
| Blood and lymphatic system disorders | Very common: anaemia, thrombocytopenia, leukocytosis |
| Nervous system disorders | Common: neurological disorders (e.g. hemiparesis, aphasia, convulsions, hemianopsia) Uncommon: brain oedema Very rare: hypaesthesia |
| Cardiac disorders | Uncommon: hypotension |
| Vascular disorders | Common: thromboembolism |
| Gastrointestinal disorders | Common: vomiting, nausea Very rare: diarrhoea |
| Hepatobiliary disorders | Very common: blood bilirubin increased, alanine aminotransferase increased, aspartate aminotransferase increased, gamma glutamyltransferase increased, blood amylase increased |
Most serious side effects include anaemia, thrombocytopenia, leukocytosis, neurological disorders and thromboembolism. Further frequently observed side effects are vomiting, nausea and increase of blood bilirubin, alanine aminotransferase, aspartate aminotransferase, gamma glutamyltransferase and blood amylase.
Ordering Gliolan®
Gliolan® is available from Alloga
Tel: +44 (0)1773 441 702
Alloga product code:
Gliolan® 1.5g (1 x 1) USP8861 – UK customers
Note: In order to ensure compliance with restrictions from the European Medicine’s Agency (EMA) that the neurosurgeon must have attended a Gliolan® training course, a Gliolan® form has to be completed and submitted with the pharmacy purchase order to info@medacpharma.co.uk
The information provided on the Gliolan® form must include the name of the qualified neurosurgeon who has successfully completed a Gliolan® training course, and a procurement pharmacy signature. This does not need to be a pharmacist.
Contact us
medac Pharma LLP,
Scion House,
Stirling University Innovation Park,
Stirling,
FK9 4NF
Tel: +44 (0)1786 458086
Email: info@medacpharma.co.uk
Medicines Information
For enquiries about Gliolan®, please contact the Medicines Information Department at medac Pharma.
Tel: +44 (0)1786 458086
Email: info@medacpharma.co.uk
Our normal enquiry service is available from 9am to 5pm Monday to Friday. However, we do offer an emergency service outside these hours for urgent enquiries.
References
- https://www.nice.org.uk/guidance/NG99.
- https://www.nice.org.uk/guidance/qs203.
- Gliolan 30mg/ml powder for oral solution – Summary of Product Characteristics (SmPC) – (emc) (medicines.org.uk).
- https://www.ema.europa.eu/en/medicines/human/EPAR/gliolan
- Stummer W et al. ALA Glioma Study Group. Fluorescence- guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006 May; 7(5):392-401.
- Stummer W et al. Prospective cohort study of radiotherapy with concomitant and adjuvant temozolomide chemotherapy
for glioblastoma patients with no or minimal residual enhancing tumour load after surgery. J Neurooncol (2012) 108:89-97. - Esteves S et al. A pilot cost-effectiveness analysis of treatments in newly diagnosed high-grade gliomas: the example of 5-aminolevulinic Acid compared with white-light surgery. Neurosurgery. 2015 May; 76(5):552-62.
- https://www.ema.europa.eu/en/documents/scientific-discussion/gliolan-epar-scientific-discussion_en.pdf
- Stummer et al, 2011